What Role Does Entropy Play in Chemical Reactions
In most cases this is established for biochemical processes 9192 or for. The role of energy.

What Triggers A Chemical Reaction Kareem Jarrah Physical Science Lessons Chemistry Classroom Teaching Chemistry
Formula relates change in enthalpy and entropy that occurs in a chemical reaction-amount of free energy change in enthalpy minus change in entropy multiplied by temperature.
. A spontaneous reaction is a reaction that occurs in a given set of conditions without intervention. What part does entropy play in chemical reactions. Energy from the breakdown of glucose and other molecules in animals is released in the form of NADP which transfers energy to other reactions.
In a closed system entropy always increases over time. A change in entropy is helpful in determining whether the reaction can proceed on its own or it requires energy. In such cases one can establish the decisive role of entropy in the free Gibbs activation energy G 89 90.
The size and direction of enthalpy the heat content of a system at constant pressure H changes and entropy changes together determine whether a reaction is spontaneous. A decrease favors the nonspontaneous reaction. How do chemical reactions play a role in energy transfer.
That is whether it. And as molecules at a high concentration in one place diffuse and spread out entropy also increases. THIS SET IS OFTEN IN FOLDERS WITH.
Terms in this set 15 How are Gibbs free energy enthalpy and entropy related. Entropy is a measure of the energy randomization or energy dispersal in a system Entropy is important because when barrier between two substances is removed the two substances mix to form a solution in which energy is spread out over a larger volume. The entropy change determines whether or not the chemical reaction is favorable.
An increase in entropy favors the spontaneous chemical reaction. In both scenarios the reaction system moves to the equilibrium point that. See full answer below.
In an open system energy can be added to a system to cause a decrease in entropy but. As chemical reactions reach a state of equilibrium entropy increases. Entropy S is a measure of the disorder in a system.
The energy released into the environmental bath is given by the difference between the free. The numerical value of ΔG is negative in spontaneous processes because the system loses free energy. What role does entropy play in chemical reactions.
17 What role does entropy play in chemical reactions. Explain the role enzymes play in chemical. Entropy S is a thermodynamic function which can be viewed as a measure of randomness or disorder and describes the number of arrangements position andor energy levels that are available to a system existing in a given state.
Entropy is measures the amount of energy that has been dispersed. A decrease favors the nonspontaneous reaction. Entropy is a measure of randomness and disorder.
What role does entropy play in chemical reactions. Whenever there is a spreading of energy there is a corresponding increase in entropy. This means that it is similar to molecules.
Energy from the breakdown of glucose and other molecules in animals is released as ATP which transfer energy to other reactions. Chemical reactions proceed to the direction where the. Entropy Changes in Chemical Reaction.
Introduce students to entropy and explore why chemical reactions happen using role play discussion and demonstrations in this lesson plan for 1618 year olds In this activity entropy is introduced to students in a qualitative way as a means. Chemical reactions the driving force is the free. Up to 10 cash back This process releases heat due to the formation of new bonds though this entropy production does not influence kddag which is given by the vibrational frequency term k_textB Th a constant for chemical reactions at temperature T.
K x 12 Br_2 g -- KBr x b. An increase in entropy favors the spontaneous chemical reaction. What role does entropy play in chemical reactions.
What is spontaneity in a reaction. Why does iodine I2 s spontaneously sublime at. Energy exists in packets called quanta.
Energy also has a role to play in the entropy or randomness of a chemical system by which we mean a quantity of substance or substances such as a reaction mixture. D The entropy change determines whether or not the chemical reaction is favorable. What two factors determine the spontaneity of a reaction.
Predict the sign of Delta S degree for each of the following changes. Entropy determines the rate of the chemical reaction and the spontaneity of the reaction. Generally a reaction will occur spontaneously if the entropy increases but the enthalpy is also negative or small and positive according to Gibbs free energy equation.
What role does entropy play in chemical reactions. 18 What is the environmentally unfriendly component of chlorinated fluorocarbons that ultimately damages ozone. An increase in entropy favors the spontaneous chemical reaction.
S sys 0 implies that the system becomes more disordered during the reaction. A biological point of view. We can apply the second law of thermodynamics to chemical reactions by noting that the entropy of a system is a state function that is directly proportional to the disorder of the system.
High entropy means high disorder and low energy. The entropy change determines whether or not the chemical reaction is favorable. You cannot have half a molecule and similarly you can have any whole number of quanta of energy but not.
A decrease favors the nonspontaneous reaction. Reaction the entropy of the surroundings decreases less at higher temperatures shifting the equilibrium to the right more towards the products. Does maximal entropy production play a role in the ev olution.
With entropy the second law of thermodynamics can be stated In any spontaneous.

Gibbs Free Energy Equilibrium Constant Enthalpy Entropy Equations Free Energy Generator Free Energy Free Energy Projects
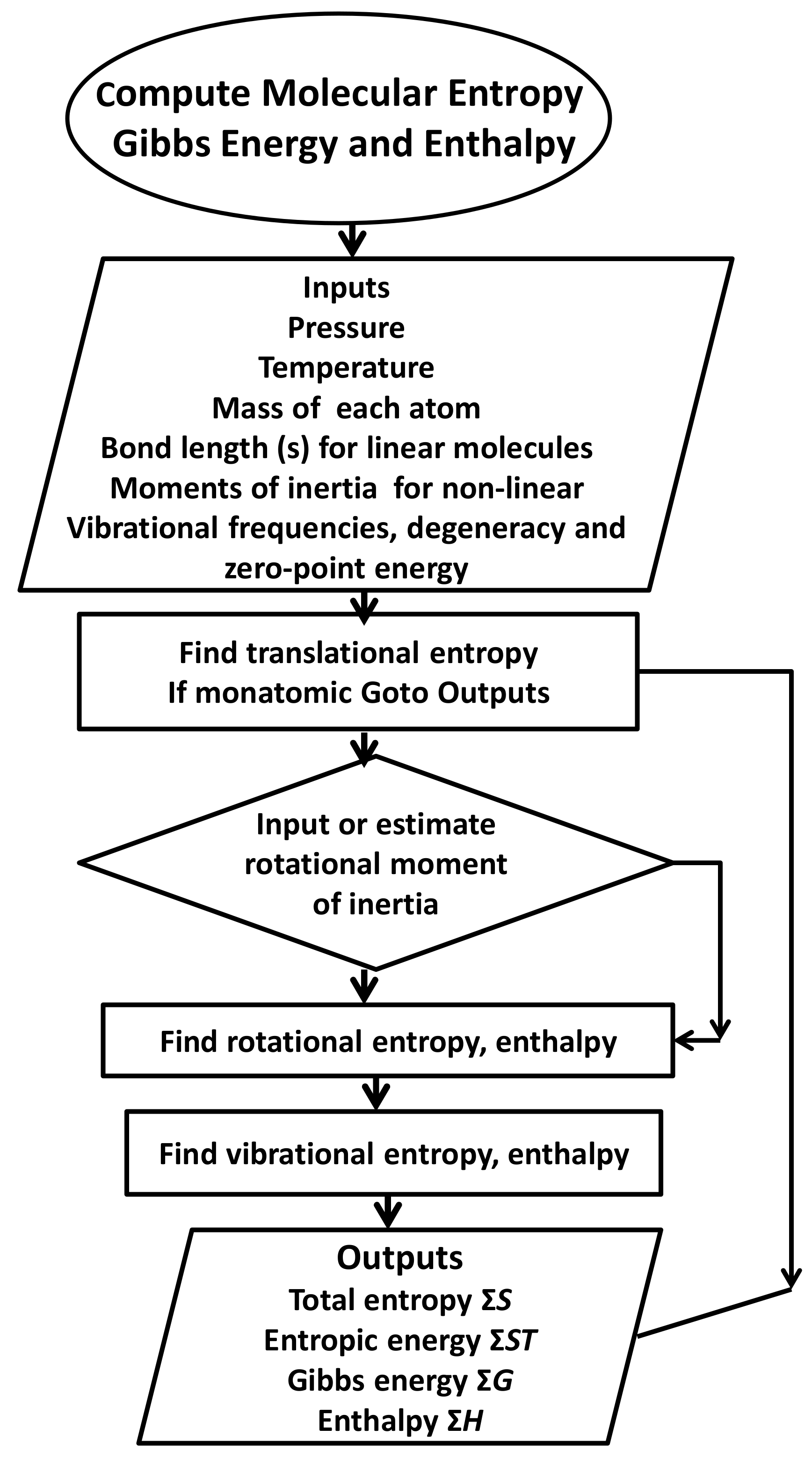
Entropy Free Full Text Partitioning Entropy With Action Mechanics Predicting Chemical Reaction Rates And Gaseous Equilibria Of Reactions Of Hydrogen From Molecular Properties Html
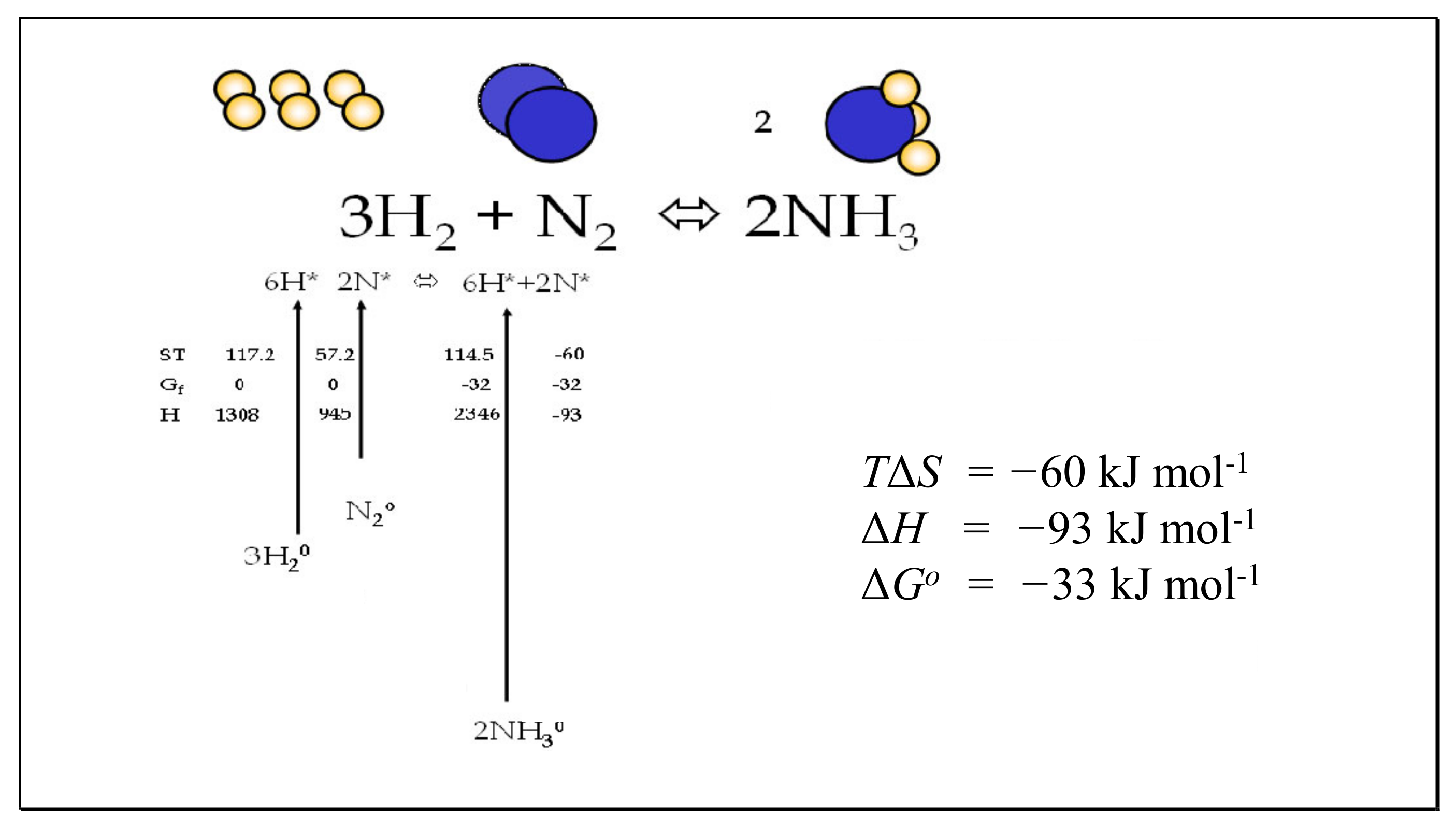
Entropy Free Full Text Partitioning Entropy With Action Mechanics Predicting Chemical Reaction Rates And Gaseous Equilibria Of Reactions Of Hydrogen From Molecular Properties Html

Mnemonic State Functions Chemistry Thermodynamics Mnemonics State Function Mcat

Chemistry Humor Chemistry Education Fun Science

Chemistry Education In Today S World Is A Challenge Which Can Be Tomorrows Future The Challenging Concepts I Chemistry Education Chemistry Chemical Education

Gibbs Free Energy Equilibrium Constant Enthalpy Entropy Equations Free Energy Generator Free Energy Free Energy Projects

Entropy Free Full Text Partitioning Entropy With Action Mechanics Predicting Chemical Reaction Rates And Gaseous Equilibria Of Reactions Of Hydrogen From Molecular Properties Html
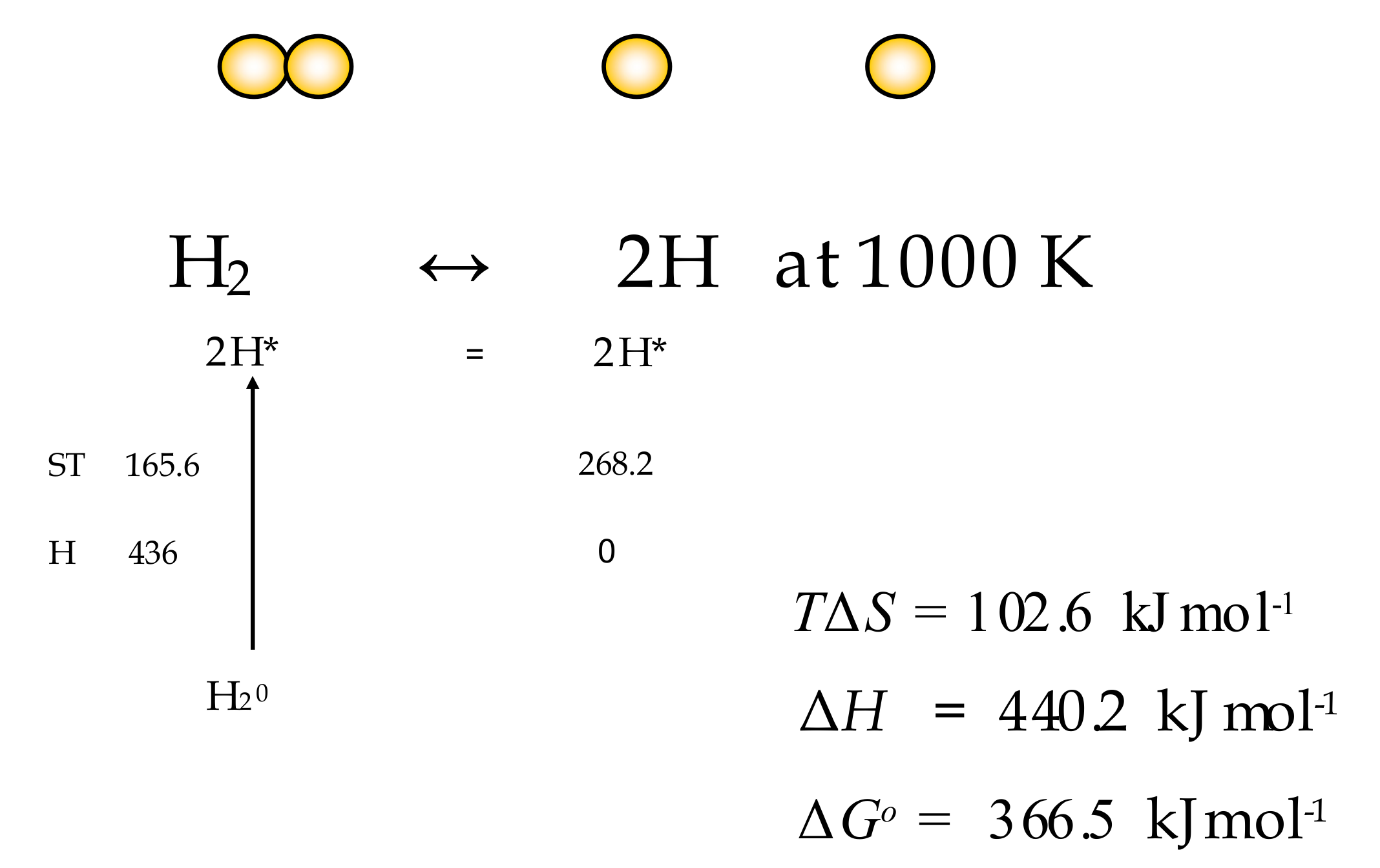
Entropy Free Full Text Partitioning Entropy With Action Mechanics Predicting Chemical Reaction Rates And Gaseous Equilibria Of Reactions Of Hydrogen From Molecular Properties Html

Chemical Thermodynamics Thermodynamics Chemical Chemistry

Applications Of Free Energy Teaching Chemistry Chemistry Classroom Chemistry Education

Entropy And Spontaneity Of The Reaction Entropy Thermodynamics Ap Chemistry
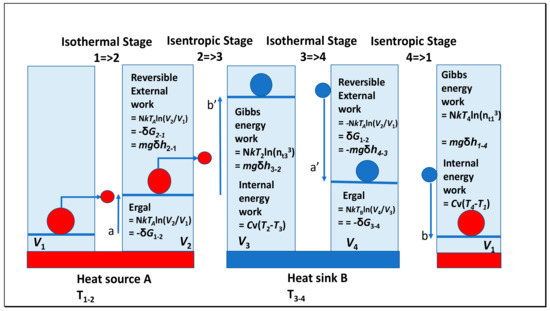
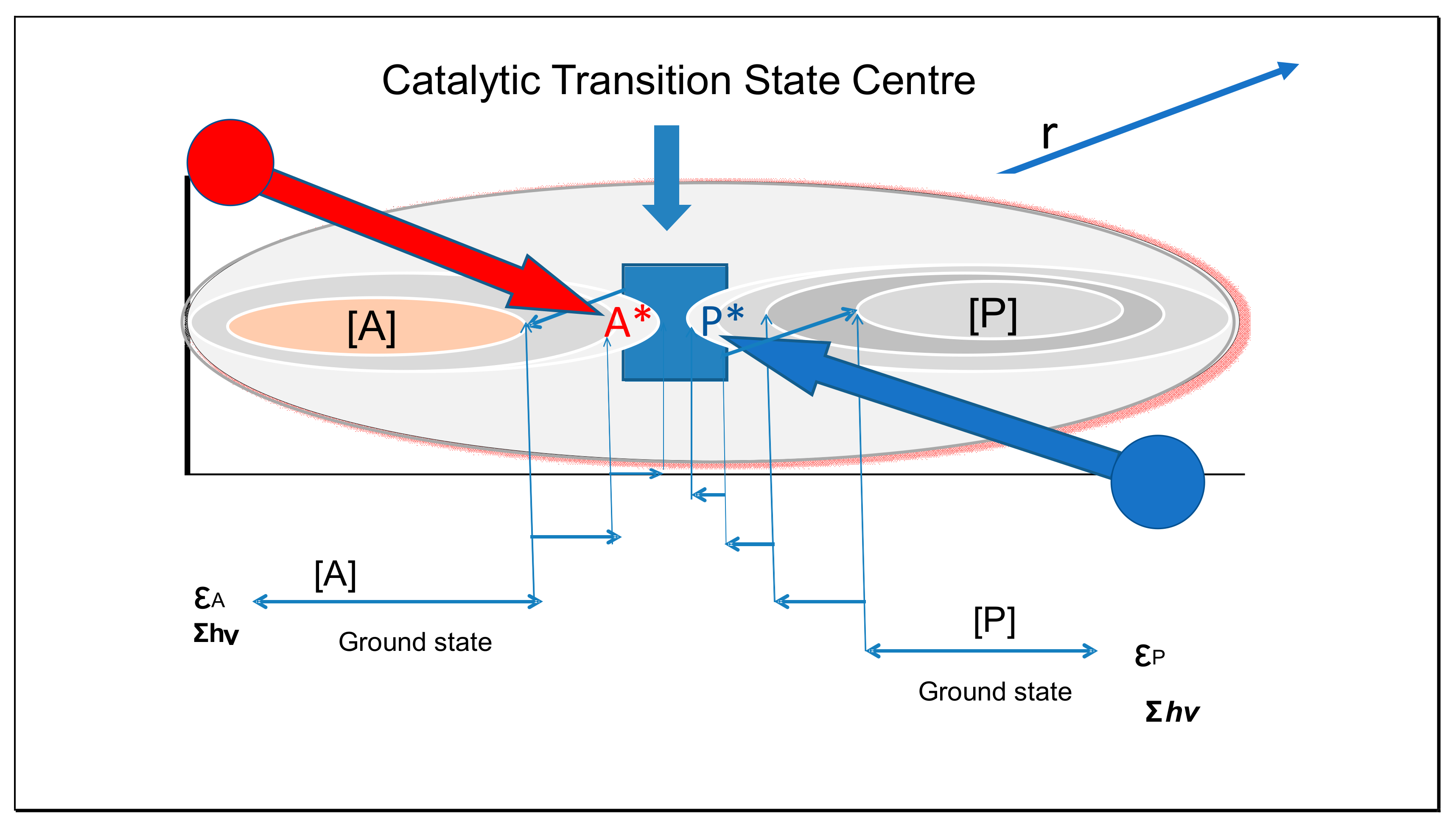
Entropy Free Full Text Partitioning Entropy With Action Mechanics Predicting Chemical Reaction Rates And Gaseous Equilibria Of Reactions Of Hydrogen From Molecular Properties Html

Thermodynamic Potentials Science Chemistry Teaching Chemistry Physics And Mathematics

What Triggers A Chemical Reaction Kareem Jarrah Physical Science Lessons Chemistry Classroom Teaching Chemistry
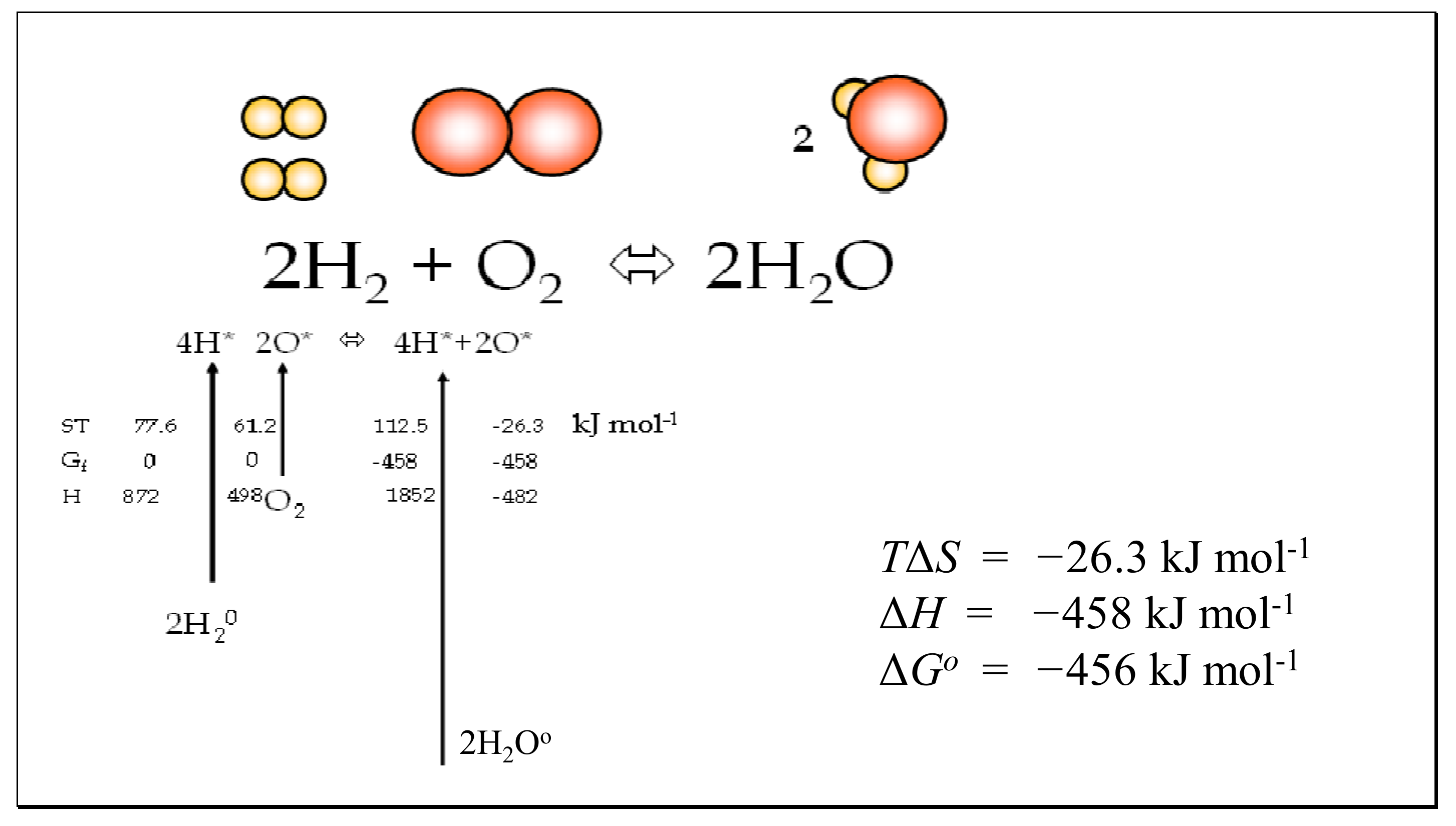
Entropy Free Full Text Partitioning Entropy With Action Mechanics Predicting Chemical Reaction Rates And Gaseous Equilibria Of Reactions Of Hydrogen From Molecular Properties Html

Thermodynamic Potentials Teaching Chemistry Science Chemistry Physical Chemistry

Using Potential Energy Diagrams Flv Physical Chemistry Potential Energy Fun Science

Comments
Post a Comment